Chorismic acid
Chorismic acid
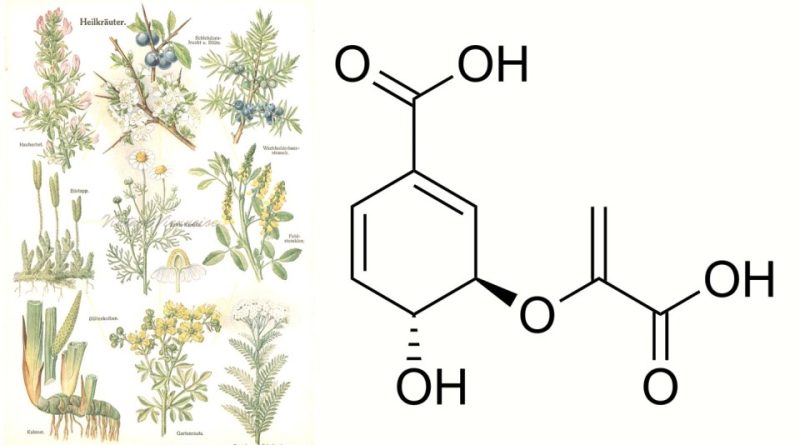
Chorismic acid, whose term in the official IUPAC nomenclature is: 3R-[(1-carboxyvinyl)oxy]-4R-hydroxycyclohexa-1,5-diene-1-carboxylic acid, is an organic acid belonging to the class of aromatic compounds .
Corismic acid has the brute or molecular formula: C10H10O6 and, from a structural point of view, it has a benzene aromatic ring with three key functional groups: a carboxyl group (-COOH) in the ortho position, a hydroxyl group (-OH) in the ortho position meta, and an isoprene side chain in the para position. This structure gives chorismic acid the ability to undergo various chemical reactions leading to the formation of complex compounds.
Chorismic acid is a basic molecule that plays a key role in the biosynthesis of various aromatic compounds, including phenols, lignins and flavonoids, within plants. This acid is an important precursor for the formation of natural chemicals that contribute to the defense of plants against pathogens, insects and environmental stresses.
Chorismic acid is involved in a number of enzymatic reactions within the metabolic pathways of plants. It is the immediate precursor of cinnamic acid and p-coumaric acid, which in turn may be involved in the synthesis of lignins and flavonoids.
Chorismic acid is most commonly known by its chorismate anionic form.
In plants it is a derivative of the shikimic acid pathway and serves as a precursor in the syntheses of the following compounds:
– aromatic amino acids phenylalanine and tyrosine;
– indole, indole derivatives and the aromatic amino acid tryptophan;
– 2,3-dihydroxybenzoic acid used in the biosynthesis of enterobactin;
– the phytohormone salicylic acid;
– many alkaloids and other aromatic metabolites.
Chorismate is transformed into para-aminobenzoic acid by the enzymes 4-amino-4-deoxychorismate synthase and 4-amino-4-deoxychorismate lyase.
Chorismate lyase is an enzyme that converts chorismate into 4-hydroxybenzoate and pyruvate. This enzyme catalyzes the first step in ubiquinone biosynthesis in Escherichia coli and other Gram-negative bacteria.
The centrality of chorismic acid in the metabolic pathway of plants has aroused the interest of researchers who study the biosynthesis of natural substances and their possible application in sectors such as agriculture, pharmacology and the food industry.
Warning: The information provided is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.