Glycosides
Glycosides
Glycosides are a very common class of chemical compounds of plant origin. From the physical point of view they are solid, colorless, soluble in water and alcohol, optically active substances.
Glycosides are synthetic substances of plants, composed of one or more sugars and a non-sugary part of various kinds.
They are highly heterogeneous products of plant metabolism and, in some cases, their exact function is not known.
However, glycosides are widely accepted and used in the pharmacological field. These include:
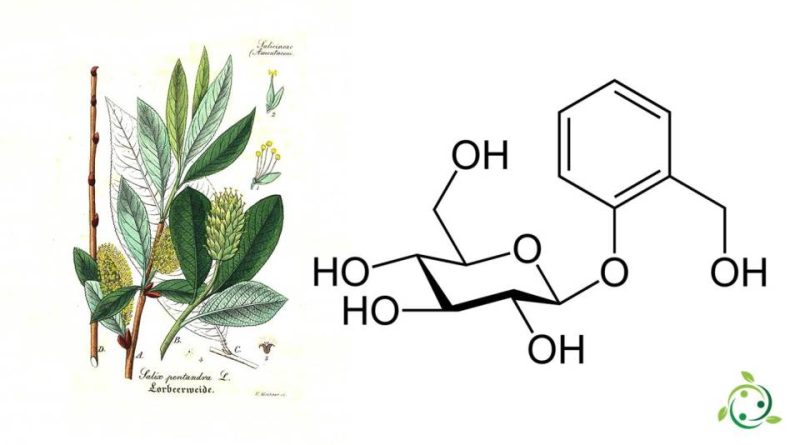
– salicylic glucosides (analgesics-antipyretics);
– anthraquinones (laxatives);
– digitalis (cardioactive);
– saponins (some anti-inflammatory, others tonic-adaptogens, other expectorants and emetics, others still precursors of cortisone);
– phenolics (urinary antiseptics);
– flavones (vasoprotective);
– etc..
Some glycosides are also of toxicological interest, such as cyanogenetic ones (they release extremely toxic hydrogen cyanide and are contained in the bitter seeds of various Rosaceae, such as the almond tree, etc.).
From a biochemical point of view, glycosides are obtained by reaction of a carbohydrate in the hemiacetal form and characterized by the formation of a glycosidic bond. The reaction is catalyzed by traces of strong acid and the resulting glycosidic carbon, which was the anomeric one of the hemiacetal, in case of reaction with an alcoholic group is bound to two different -OR groups. The sugary part of these compounds is called glycone while the non-sugary part is called aglycone.
A glycoside is stable in aqueous solution at neutral or basic pH, without presenting equilibria with anomeric forms. In an acidic environment or by the action of specific hydrolase enzymes, the glycosidic bond is split with the formation of hemiacetal anomers.
Furthermore, at the biochemical level, the glycosidic bonds are in fact split by glycosidases, enzymes with a lysosomal localization belonging to the class of hydrolases. The hereditary deficiency of β-1,6-glycosidase causes, in humans, an alteration of the metabolism of glycolipids which is the basis of the disease known as Gaucher’s cerebrosidosis.
Warning: The information shown is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.