Tannic Acid
Tannic Acid
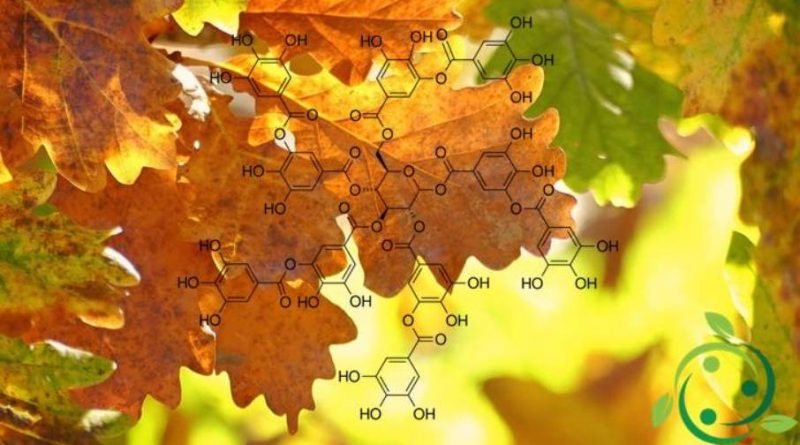
Tannic acid, whose name in the IUPAC nomenclature is: 2,3-dihydroxy-5 – ({[(2R, 3R, 4S, 5R, 6R) -3,4,5,6-tetrakis ({3,4 – dihydroxy-5 – [(3,4,5-trihydroxyphenyl) carbonyloxy] phenyl} carbonyloxy) oxan-2-yl] methoxy} carbonyl) phenyl 3,4,5-trihydroxybenzoate has brute or molecular formula: C76H52O46.
Tannic acid is formed by the condensation of a glucose molecule and five digallic acid molecules, in turn formed by esterification of two molecules of gallic acid.
The phenolic groups present in the molecular structure give the tannic acid a slight acidity (pKa 10). Tannic acid reacts with strong bases to sleep salts called tannins.
Furthermore, tannic acid is the form in which tannin, a polyphenol, is marketed.
Tannic acid is produced in nature and is present in oak, walnut and mahogany woods and is used as a mordant for cellulose fibers, such as cotton.
Tannic acid has been used historically against strychnine poisoning between the 19th and 20th centuries.
Today, this substance, used in various pharmaceutical aids and used in ointments for the treatment and application of various symptoms, is also used to kill various mites in domestic environments.
In large quantities, tannic acid can cause side effects such as stomach irritation, nausea, vomiting and liver damage. Regular consumption of herbs with high concentrations of tannins seems to be associated with a greater probability of developing some carcinogenic activities.
Tannic acid is not dangerous for the environment.
Warning: The information given is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.