Sterculic acid
Sterculic acid
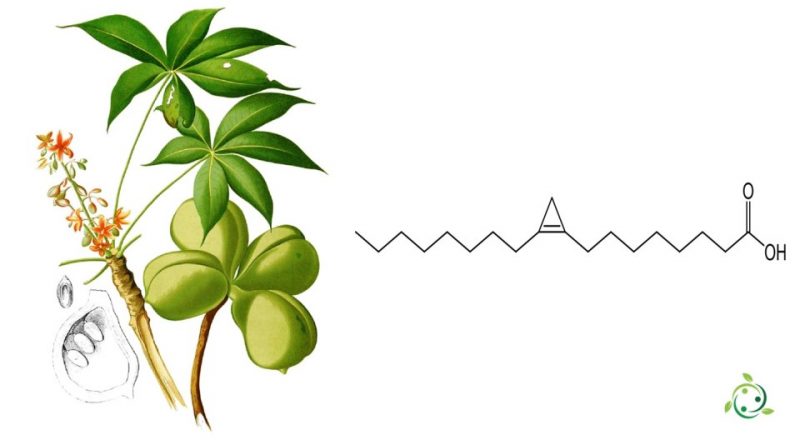
Sterculic acid, whose term in the official IUPAC nomenclature is 8-(2-octylcyclopropen-1-yl)octanoic acid, is a fatty acid composed of 19 carbon atoms with a cyclopropene ring, i.e. a cyclopropane group with a double bond , in position 9-10.
Sterculic acid was first isolated in 1952 by JR Nunn from the seed oil of Sterculia foetida.
Sterculic acid is a substance of natural origin as it is also found in high concentrations in the carbohydrates of the seed oils of various Sterculiaceae plants, from which it takes its name, Malvaceae, Bombacaceae.
It is mainly present in vegetable oils such as the seed oil of Sterculia foetida, a tropical plant.
From a physical point of view, sterculic acid is solid at room temperature. It has a melting point of approximately 56-58°C.
From a chemical point of view it is susceptible to the hydrogenation reaction, which can transform it into a saturated fatty acid. It is also subject to other chemical reactions typical of unsaturated fatty acids, such as oxidation.
Sterculic acid has attracted interest due to its potential health-promoting properties. Preliminary studies suggest it may have positive effects on reducing cholesterol and preventing heart disease, although more research is needed to confirm these benefits.
In addition to its possible health benefits, sterculic acid also has industrial applications, for example in the food and pharmaceutical industries.
Like other cyclopropene-containing fatty acids, it is considered toxic. Seed oils containing it can only be edible if subjected to refining to reduce their content.
Seed oils that contain sterculic acid come from: Durio zibethinus (39-78%), Sterculia foetida (44–65%), Bombacopsis glabra (34-64%), Bombax oleagineum (≈30%); Bombacopsis glabra (≈30%), Gnetum scandens (≈29%), Ceiba pentandra (≈3%). Cottonseed oil (Gossypium hirsutum and Gossypium herbaceum) and baobab oil (Adansonia digitata) which may have food use if unrefined may contain concentrations of ≤2% and ≤8% respectively. It has also been detected in spoiled walnut oil. In vegetable oils it is almost always associated with the homologue malvalic acid, 8,9-cpe-18:1, its probable derivative by α-oxidation.
From the point of view of its biochemical functions in plants, it is believed that sterculic acid, like other cyclopropenoid fatty acids, has a protective function against predators and pathogens of the plants that produce it, as this substance interferes with the metabolism of fats in processes that implement the insertion of a double bond. In particular, sterculic acid would inhibit the action of the desaturase enzymes Δ5, Δ6 and Δ9. This results in a changed composition of body fat in animals, which affects not only the accumulated fat, but also the lipid composition of the membranes.
It should be underlined that the melting point increases due to the increase in the saturated/unsaturated ratio. The presence, for example, of sterculic acid in the feed of laying hens, leads to a clear change in the color of the eggs even at a daily dose of 25 mg. In these concentrations the egg white takes on a pink color, while the yolk appears apricot in color. This is due to increased permeability of the membrane separating the yolk from the albumen, which leads to the transfer of the protein conalbumin from the albumen to the yolk. There, a pink complex is formed with the iron present in the yolk, which in turn can diffuse into the egg white.
The intake of sterculic acid in animals has different consequences, such as, for example, a reduction in growth and their reproductive function.
Warning: The information provided is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.