Mimosine
Mimosine
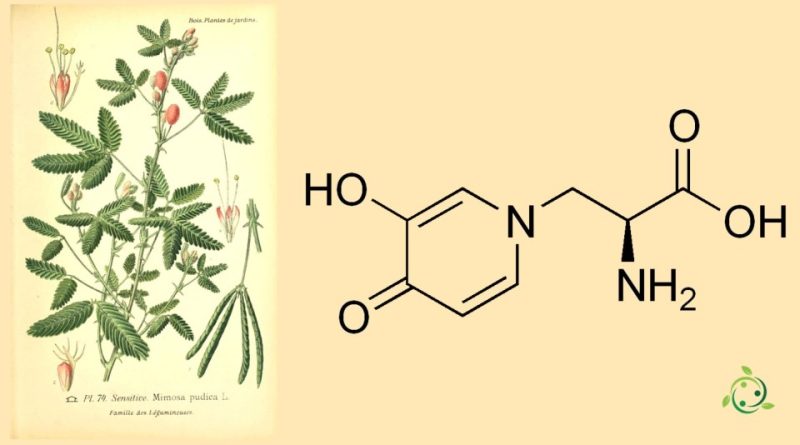
Mimosine, whose term in the IUPAC nomenclature is: (2S)-2-Amino-3-(3-hydroxy-4-oxopyridin-1-yl)propanoic acid is a toxic amino acid with the brute or molecular formula: C8H10N2O4.
From a physical point of view, the hydrochloride salt melts between 174.5 and 175.0 °C, with decomposition; hydrobromide decomposes at 179.5°C and hydroiodide decomposes between 183.0 and 183.5°C. Mimosine forms only monobasic acids, but the methyl ester forms a dihydrochloride, C7H9O2N2(COOMe)•2 HCl•½ H2O, mp. 175–6°C.
Mimosine is a chemical found in some plants, most notably Mimosa pudica, a plant known for its touch-sensitive leaves that close when touched. Mimosine is a non-protein amino acid and contains a structure similar to the amino acid tyrosine. However, mimosine is toxic to many animals, including humans, due to the presence of a toxic compound called 3,4-dihydroxyphenylalanine (DOPA).
In some contexts, mimosine has been used in scientific studies to induce cellular stress and programmed cell death in animal and human cells. However, due to its toxicity, the use of mimosine is limited and should be approached with caution.
This compound, also known as leukenol, was first isolated from the seeds of Leucaena glauca Benth., and was subsequently studied by Adams and colleagues.
Mimosine arrests cell division in the late G1 phase by inhibiting the initiation of DNA replication. In ruminants, mimosine is degraded to 3,4- and 2,3-dihydroxypyridone (3,4- and 2,3-DHP).
Although toxicosis has been found in Australia, Papua New Guinea, Africa and Florida, it has not been found in any other tropical and subtropical regions. Goats in Burma lost their hair when fed a diet containing 50% Leucaena. Goats and cattle in Hawaii are capable of degrading 3,4-DHP rumenally. Tolerance could be related to the presence or absence of microbes tolerant to mimosine and 3,4-DHP. It is known that at least Australian goats do not share the abilities of their Hawaiian counterparts.
Researchers Bickel and Wibaut found, in feeding experiments with rats and mice, that mimosine is likely the toxic constituent of Leucaena glauca seeds, but they did not observe the hair loss in these animals that appears to occur when these seeds are fed. to livestock.
Some rhizobia are known to produce rhizomimosinase, which consumes pyridoxal 5′-phosphate to degrade mimosine into 3,4 dihydroxypyridine, pyruvate, and ammonium. A similar enzyme, mimosinase, is produced by Leucaena leucocephala.
It is important to note that although mimosine is present in some plants, it should not be consumed or used without due precaution due to its toxic health effects.
Warning: The information provided is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.