Phorbol
Phorbol
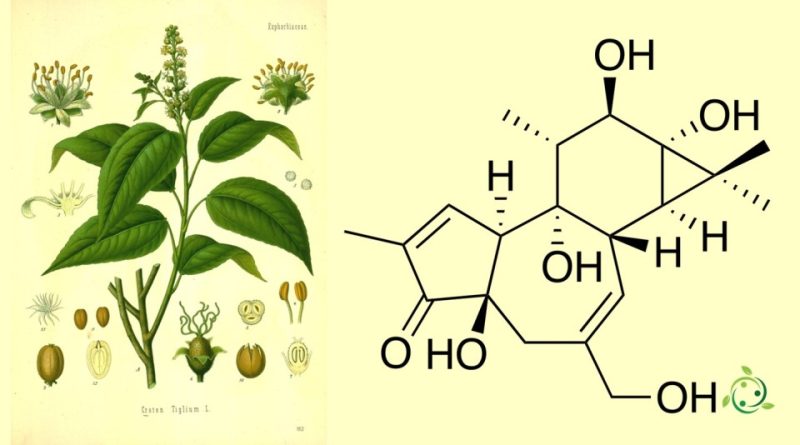
Phorbol, whose term in the official IUPAC nomenclature is: (1aR,1bS,4aR,7aS,9bS,8R,9R,9aS)-4a,7b,9,9a-Tetrahydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-1,1a,1b,4,4a,7a,7b,8,9,9a-decahydro-5H-cyclopropa[3,4]benzo[1,2-e]azulen-5-one, is a natural organic compound of plant origin with brute or molecular formula: C20H28O6.
Phorbol belongs to the tiglian diterpene family.
This substance was first isolated in 1934 as a hydrolysis product of croton oil, which comes from the seeds of Croton tiglium.
The structure of phorbol was determined only in 1967.
Phorbol forms various esters that have important biological properties, the most notable of which is the ability to act as tumor promoters through the activation of protein kinase C. They mimic diacylglycerols, glycerol derivatives in which two hydroxyl groups have reacted with acids fats to form esters. The most common and potent phorbol ester is 12-O-tetradecanoylphorbol-13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical research tool in settings such as models of carcinogenesis.
In nature, phorbol is a substance present in many plants, particularly those of the Euphorbiaceae and Thymelaeaceae families.
Furthermore, phorbol is the active constituent of the tropical mancinella (Hippomane mancinella L.), a highly toxic plant.
This substance is very soluble in most polar organic solvents, as well as in water.
However, Croton tiglium is the source of the oil from which phorbol was initially isolated. Its seeds and oil have been used for hundreds of years in traditional medicine, generally as a purgative, and the seeds were mentioned in Chinese herbal texts 2,000 years ago.
The purgative effects of the oil are largely attributed to the high percentage of phorbol esters contained in the oil.
Phorbol can also be obtained by synthesis, although this synthesis will not replace natural isolation products, it will allow researchers to create phorbol analogues for use in research, in particular by creating phorbol derivatives that can be evaluated for anti-tumor activity.
Previously, the difficulty in synthesizing phorbol had been the creation of C–C bonds, especially in the six-membered ring at the top of the molecule. This synthesis starts from (+)-3-carene and uses a series of 19 steps to ultimately create (+)-phorbol.
Warning: The information provided is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.