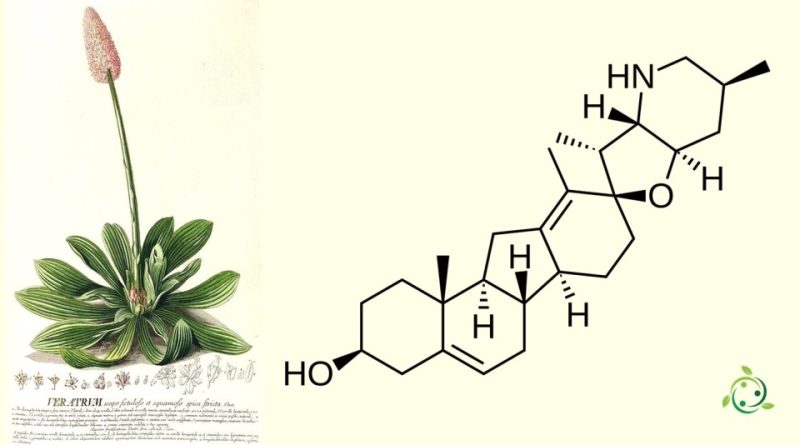
Cyclopamine
Cyclopamine
Cyclopamine is a natural chemical compound, whose term in the official IUPAC nomenclature is: (2′R,3S,3′R,3′aS,6′S,6aS,6bS,7′aR,11aS,11bR)-3′,6′,10,11b-Tetramethyl-1,2,3,3′a,4,4′,5′,6,6′,6a,6b,7,7′,7′a,8,11,11a,11b-octadecahydro-3′H-spiro[benzo[a]fluorene-9,2′-furo[3,2-b]pyridin]-3-ol.
Cyclopamine, or 11-deoxojervina, has the chemical formula: C27H41NO2.
This substance belongs to the class of steroid alkaloids and was first isolated from the plant Veratrum californicum, also known as American hellebore. This plant is toxic and grows mainly in certain regions of North America.
Cyclopamine is known for its teratogenic properties, which means that it can cause abnormalities in embryonic development if taken during pregnancy. It has been studied primarily for its potential use in biomedical research, particularly in the study of embryonic development and tissue formation. It has also been the subject of research in molecular and developmental biology as it may influence cell signaling and impact tissue and organ formation during fetal development.
This substance prevents the embryonic brain from separating into two lobes (an extreme form of holoprosencephaly), which in turn causes the development of a single eye (cyclopia). The chemical gets its name from this effect, as was originally noted by Idaho lamb farmers who contacted the United States Department of Agriculture after their herds gave birth to cyclops lambs in 1957. It took more than a decade to identify Veratrum californicum as responsible for this effect.
The venom disrupts the hedgehog’s sonic signaling pathway during development, thus causing birth defects.
Cyclopamine was one of three steroidal alkaloids isolated from Veratrum californicum, but the only one unknown at the time, and got its name from its effects on sheep embryos.
Cyclopamine biosynthesis begins with cholesterol. A steroid skeleton has a classic 6-membered ring, adjacent to another 6, 6, then a five or “6-6-6-5”. Veratrum has been determined to contain five types of alkaloids, each of which had a common cholesterol precursor: solanidine alkaloid, verazine alkaloid, vertramine alkaloid, jervina alkaloid, and cevanine alkaloid. In biosynthesis, cyclopamine has a precursor solanide, which in turn consists of cholesterol. This was brought about by initial studies which isolated the alkaloids from Veratrum californium and introduced them into embryonic ewes. At the time, jervina was an alkaloid already known which was isolated from the corn lily together with two other alkaloids: the unknown cyclopamine and veratramine; each with different toxicities. Subsequent studies then showed that jervin degrades to cyclopamine following a Wolff-Kishner reduction, which helped identify the unknown compound.
Treatment of cyclopamine with a Lewis acid (pH < 2) has also been shown to lead to the production of veratramine. The stomach provides these conditions and therefore only a small amount of cyclopamine passes through the stomach after ingestion. And even if only a small amount of ingested cyclopamine passes through the stomach, it remains unaffected afterwards. Veratramine is highly toxic, although it does not affect development, as it excites the central nervous system and can cause seizures, similar to serotonin. The mechanism for the production of veratramine focuses on the cleavage of a carbon-oxygen bond, which leads to the formation of a new double bond. From there, this ring is almost aromatic. The driving force to become aromatic then drives an elimination reaction, creating a third double bond and producing aromaticity, as illustrated in the following figure. Interestingly, cyclopamine has also been studied for its potential to inhibit the Hedgehog (Hh) signaling pathway which is involved in various developmental processes and, when assimilated, may contribute to various diseases, including some forms of cancer.
Therefore, cyclopamine and its synthetic variants have been investigated as potential therapeutic agents for the treatment of diseases related to the abnormal regulation of the Hh pathway, such as basal cell carcinoma. Cyclopamine is currently being studied as a therapeutic agent in basal cell carcinoma, medulloblastoma and rhabdomyosarcoma, which are tumors resulting from excessive Hh,3 activity as well as in glioblastoma and multiple myeloma. In summary, cyclopamine is a naturally occurring chemical that has aroused the interest of the scientific community due to its role in embryonic development and its potential implications in the research and therapy of certain diseases.
Warning: The information provided is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.