Mugineic acid
Mugineic acid
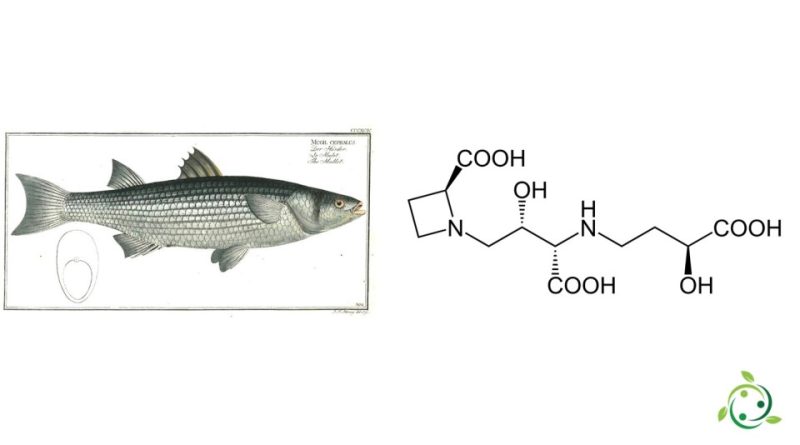
Mugineic acid whose term in the official IUPAC nomenclature is: N-(3-(3-carboxypropylamino)-2-hydroxy-3-carboxypropyl)azetidin-2-carboxylic acid, is an organic compound belonging to the carboxylic acid family.
Mugineic acid has a brute or molecular formula: C10H20N2O8 and was initially isolated from the mullet fish (Mugil cephalus Linnaeus, 1758), from which its name derives. Mugineic acid is also present in grasses.
Mugineic acid is a medium length phytochelatin, consisting of three amino acids: glutamic acid, glycine and methionine. These amino acids are linked together via peptide bonds, forming a chain structure. Mugineic acid is capable of binding metal ions such as iron (Fe) and copper (Cu) through amino groups present in its constituent amino acids.
The main function of mugineic acid is to act as a natural chelator, i.e. a compound that forms stable complexes with metal ions. In plants, mugineic acid plays an important role in the transport and absorption of essential metal ions such as iron. In fact, mugineic acid can form complexes with iron in the soil surrounding plant roots, increasing its availability for root uptake. Furthermore, mugineic acid is involved in the regulation of gene expression of some genes involved in iron metabolism.
In addition to its role in iron metabolism, mugineic acid may also perform other biological functions. Recent studies have suggested a possible involvement of mugineic acid in the response of plants to environmental stress, such as oxidation and heat.
In detail we see some of the functions of mugineic acid:
– Chelator of iron: MA has a strong affinity for iron and can form stable complexes with this metal. This chelating property allows AM to facilitate the uptake and transport of iron in plants, which is essential for the synthesis of chlorophyll and other proteins involved in photosynthesis.
– Regulation of iron metabolism: MA acts as a molecular signal to regulate gene expression involved in iron metabolism. It stimulates the expression of genes coding for proteins involved in the absorption and transport of iron, thus increasing the efficiency of the absorption of this nutrient by plant roots.
– Induction of phytosiderophore synthesis: MA stimulates the synthesis and secretion of compounds called phytosiderophores, which are high molecular weight organic compounds that bind iron and facilitate its absorption. Phytosiderophores are produced by plant roots and help to solubilize and transport insoluble iron present in the soil.
– Improved iron tolerance: MA is involved in regulating the response of plants to iron excess or iron deficiency. MA-treated plants show an increase in iron tolerance under deficient conditions and a decrease in damage caused by excess iron.
– Interactions with other nutrients: MA can also interact with other nutrients such as zinc, copper and manganese, facilitating their absorption and transport in plants.
These are just some of the main properties of mugineic acid. Research on MA and its role in iron metabolism and plant physiology is still ongoing, and further studies are needed to fully understand its impact and potential applications in agriculture and biotechnology.
Warning: The information provided is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.