Bifaline
Bifaline
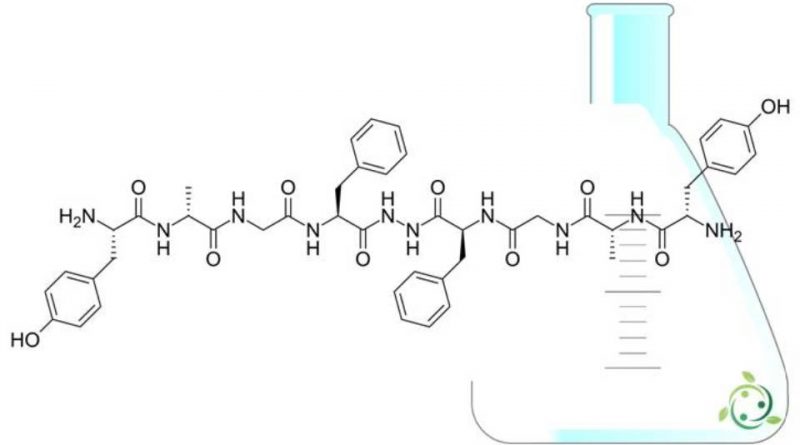
Bifaline is a dimeric opioid peptide (Tyr-D-Ala-Gly-Phe-NH) 2, consisting of two identical enkephalin-related tetrapeptides connected from tail to tail through a hydrazine bridge.
In the official IUPAC nomenclature, bifaline takes the following term: (2S, 2’S) -N, N ‘- [(2R, 8S, 13S, 19R) -8,13-Dibenzyl-3,6,9,12,15, 18-hexoxy-4,7,10,11,14,17-hexaazaicosane-2,19-dil] bis [2-amino-3- (4-hydroxyphenyl) propanamide].
Bifaline has a brute or molecular formula: C46H56N10O10.
In bifaline the presence of two distinct pharmacophores gives this molecule a high affinity for both the μ and the δ opioid receptors (with an EC50 for the two receptors of about 1-5 nM), thus showing analgesic activity.
Bifaline also exhibits an antinociceptive profile worthy of consideration.
By administering bifaline to mice intracerebroventricularly, it shows a capacity 7 times greater than the potent opioid agonist etorphine and 7,000 times greater than morphine; it should also be noted that bifaline and morphine proved to be equipotent following interperitoneal administration.
Furthermore, the effective capacity of this molecule, in vivo, is accompanied by reduced side effects, especially as regards the appearance of addiction following prolonged intake.
This is why many studies have been carried out in order to obtain more information on structure-activity relationships (SARs).
The results have led to the view that, at least for binding to the μ-receptor, the presence of two pharmacophores is not necessary.
It is emphasized that Tyr1 is indispensable for analgesic activity, while the replacement of Phe in 4 and 4 ‘with non-aromatic but lipophilic amino acids does not particularly influence receptor affinity; in general, the 4,4 ‘position is considered important for the realization of higher power analogues and to modulate the selectivity δ / μ. The hydrazine linker is not essential for activity and affinity, and can be conveniently replaced with various cycloaliphatic substituents with higher conformational rigidity.
Warning: The information given is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.