Siderophore
Siderophore
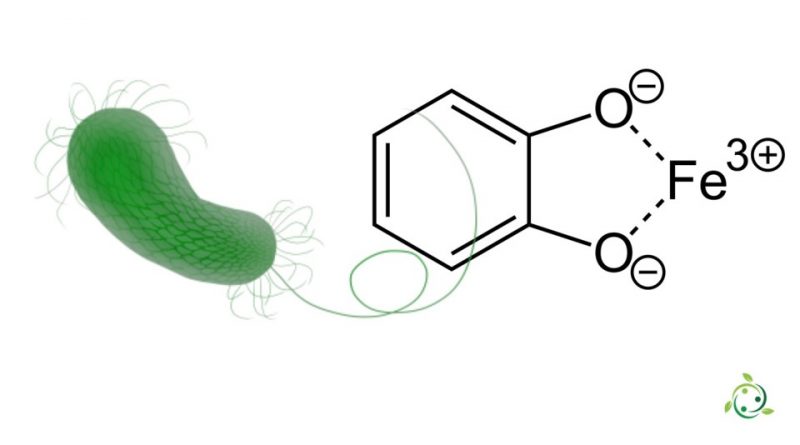
The term siderophore refers to molecules or cellular structures that are involved in the transport and absorption of iron ions (Fe^2+ and Fe^3+) within living organisms. Iron is an essential element for many vital biological reactions, such as cellular respiration and hemoglobin synthesis. In bacteria, for example, siderophores are often secretions of small organic molecules that bind tightly to iron and transport it into bacterial cells. In mammals, iron transporter proteins such as transferrin play a similar role in the transport of iron through the blood.
Siderophores can be produced by a wide range of living organisms, including bacteria, fungi, plants, and even animals. They are often released into the surrounding environment by bacteria and fungi to capture and absorb available iron in the soil or aquatic environment. Plants can secrete siderophores from their roots to facilitate the absorption of iron from the soil.
In mammals, iron transporter proteins, such as transferrin in the blood or ferritin within cells, perform similar functions in iron transport and storage. Although they are not called siderophores, these proteins play a crucial role in maintaining iron homeostasis in higher organisms.
In the natural environment, the species that produce siderophores are mainly bacteria and fungi. Some examples of bacteria that produce siderophores are:
1. Pseudomonas spp.
2. Bacillus spp.
3. Azotobacter spp.
4. Rhizobium spp.
5. Streptomyces spp.
Among fungi, some mycorrhizal fungi can also produce siderophores to facilitate the absorption of iron by the plants with which they establish a symbiosis. These include:
1. Ectomycorrhizal fungi such as the genera Rhizopogon and Suillus.
2. Endomycorrhizal fungi such as the genus Glomus.
These microorganisms produce siderophores as part of their strategies to compete with other organisms for iron in the soil and to provide iron to the host plants with which they interact.
In short, siderophores can be found in a variety of environments, from plant roots to soil, from the aquatic environment to body fluids in animals, where they play a critical role in ensuring access to the iron needed for life and growth of organisms.
Regarding the structure, siderophores usually form a stable, hexadentate, octahedral complex preferentially with Fe3+ compared to other abundant metal ions present in nature. The most effective siderophores are those that have three bidentate ligands per molecule, forming a hexadentate complex and causing less entropic change than that caused by single ferric ion chelators with separate ligands.