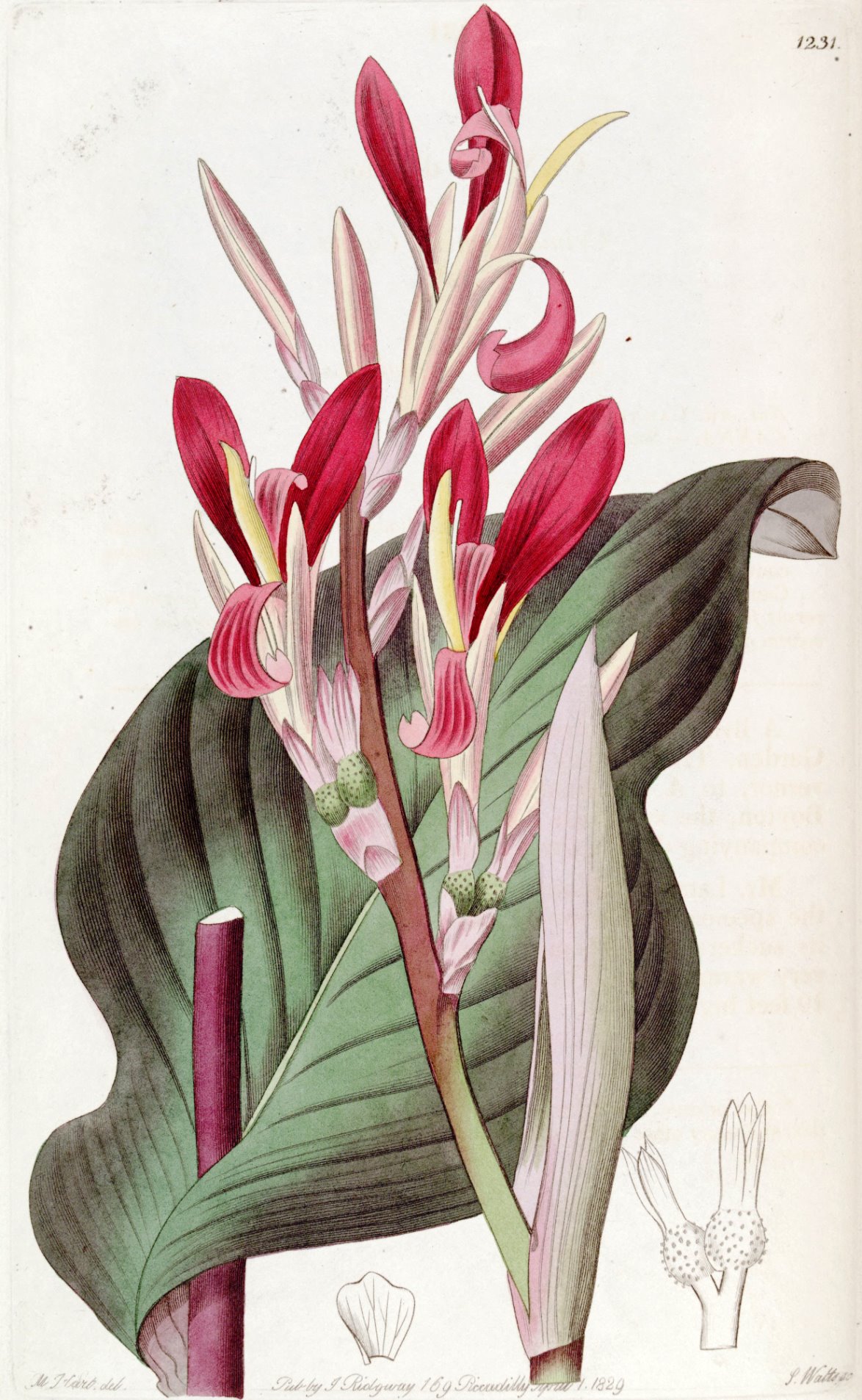
Canna indica
Canna indica
The Indian shot or African arrowroot, edible canna, purple arrowroot, Sierra Leone arrowroot (Canna indica L., 1753) is a herbaceous species belonging to the Cannaceae family.
Systematics –
From a systematic point of view it belongs to:
Eukaryota domain,
Kingdom Plantae,
Magnoliophyta division,
Class Liliopsida,
Subclass Zingiberidae,
Zingiberales Order,
Cannaceae family,
Genus Reed,
Species C. indica.
The terms are synonyms:
– Canna achiras Gillies;
– Canna achiras Gillies ex D.Don;
– Canna altensteinii Bouché;
– Canna amabilis T.Koyama & Nob.Tanaka;
– Canna ascendens Ciciar.;
– Canna atronigricans André;
– Canna aurantiaca Roscoe;
– Canna aurantiaca subsp. splendida Année;
– Canna aurantiaca subsp. splendida Année ex André;
– Canna aureovittata G.Lodd.;
– Canna barbadica Bouché;
– Canna bidentata Bertol.;
– Canna bifida Roem. & Schult.;
– Canna bihorellii G.Nicholson;
– Canna brasiliensis Roscoe;
– Canna brasiliensis Roscoe ex Spreng.;
– Canna caledonis-peltata Chaté;
– Canna carnea Roscoe;
– Canna cearensis Huber;
– Canna chinensis Willd.;
– Canna cinnabarina Bouché;
– Canna coccinea Mill.;
– Canna coccinea f. flaviflora Chodat & Hassl.;
– Canna coccinea subsp. bicolor Kraenzl.;
– Canna coccinea subsp. concolor Regel;
– Canna coccinea subsp. flaviflora Chodat & Hassl.;
– Canna coccinea subsp. floribunda (Bouché) Regel;
– Canna coccinea subsp. limbata Regel;
– Canna coccinea var. bicolor Kraenzl.;
– Canna coccinea var. concolor Regel;
– Canna coccinea var. floribunda (Bouché) Regel;
– Canna coccinea var. limbata Regel;
– Canna commutata Bouché;
– Canna compacta Bouché;
– Canna compacta Roscoe;
– Canna concinna Bouché;
– Canna crocea Lag.;
– Canna crocea Lag. ex Rchb.;
– Canna crocea Roem. & Schult.;
– Canna densiflora Bouché;
– Canna densifolia Bouché;
– Canna denudata var. grandis Petersen;
– Canna discolor Lindl.;
– Canna discolor subsp. rubripunctata Nob.Tanaka;
– Canna discolor subsp. viridifolia Nob.Tanaka;
– Canna discolor var. rubripunctata Nob.Tanaka;
– Canna discolor var. viridifolia Nob.Tanaka;
– Canna edulis Ker Gawl.;
– Canna edulis KerGawl.;
– Canna ehrenbergii Bouché;
– Canna elegans Raf.;
– Canna ellipticifolia Stokes;
– Canna ellipticifolia subsp. coccinea (Mill.) Stokes;
– Canna ellipticifolia subsp. lutea (Mill.) Stokes;
– Canna ellipticifolia subsp. patens (Aiton) Stokes;
– Canna ellipticifolia subsp. rubra Stokes;
– Canna ellipticifolia var. coccinea (Mill.) Stokes;
– Canna ellipticifolia var. lutea (Mill.) Stokes;
– Canna ellipticifolia var. patens (Aiton) Stokes;
– Canna ellipticifolia var. rubra Stokes;
– Canna esculenta G.Don;
– Canna esculenta Loudon;
– Canna exigua Bouché;
– Canna eximia Bouché;
– Canna eximia Bouché ex Horan.;
– Canna expansa Année;
– Canna expansa Année ex André;
– Canna expansa-rubra G.Nicholson;
– Canna flavescens Link;
– Canna floribunda Bouché;
– Canna formosa Bouché;
– Canna fuchsina Ciciar.;
– Canna fulgida Bouché;
– Canna gaboniensis Chaté;
– Canna heliconiifolia Bouché;
– Canna heliconiifolia subsp. xalapensis Kraenzl.;
– Canna heliconiifolia var. xalapensis (Bouché) Kraenzl.;
– Canna houlletii André;
– Canna humilis Bouché;
– Canna indica f. rubroaurantiaca Makino;
– Canna indica subsp. coccinea (Mill.) Aiton;
– Canna indica subsp. edwardsii Regel;
– Canna indica subsp. edwarsii Regel;
– Canna indica subsp. flava (Roscoe) Baker;
– Canna indica subsp. karsteniana Regel;
– Canna indica subsp. limbata (Regel) Petersen;
– Canna indica subsp. lutea (Mill.) Aiton;
– Canna indica subsp. maculata Hook.;
– Canna indica subsp. nepalensis (Bouché) Baker;
– Canna indica subsp. orientalis Baker;
– Canna indica subsp. patens Aiton;
– Canna indica subsp. rubra Aiton;
– Canna indica subsp. rubroaurantiaca Makino;
– Canna indica subsp. sanctae-rosae (Kraenzl.) Nob.Tanaka;
– Canna indica subsp. speciosa Baker;
– Canna indica subsp. variegata Regel;
– Canna indica subsp. warszewiczii (A.Dietr.) Nob.Tanaka;
– Canna indica var. coccinea (Mill.) Aiton;
– Canna indica var. coccinea Willd.;
– Canna indica var. edwardsae Regel;
– Canna indica var. edwardsii Regel;
– Canna indica var. flava (Roscoe) Baker;
– Canna indica var. indica;
– Canna indica var. karsteniana Regel;
– Canna indica var. limbata (Regel) Petersen;
– Canna indica var. lutea (Mill.) Aiton;
– Canna indica var. maculata Hook.;
– Canna indica var. nepalensis (Bouché) Baker;
– Canna indica var. orientalis Baker;
– Canna indica var. patens Aiton;
– Canna indica var. rubra Aiton;
– Canna indica var. sanctae-rosae (Kraenzl.) Nob.Tanaka;
– Canna indica var. saturaterubra Regel;
– Canna indica var. speciosa Baker;
– Canna indica var. variegata Regel;
– Canna indica var. warszewiczii Nob.Tanaka;
– Canna insignis G.Nicholson;
– Canna juncea Retz.;
– Canna laeta Bouché;
– Canna lagunensis Lindl.;
– Canna lambertii Lindl. ex Ker Gawl.;
– Canna lanuginosa Roscoe;
– Canna lavallei André;
– Canna leptochila Bouché;
– Canna liervalii André;
– Canna limbata Roscoe;
– Canna limbata var. hybrida Année;
– Canna limbata var. hybrida Année ex André;
– Canna lutea Larrañaga;
– Canna lutea Mill.;
– Canna lutea subsp. aurantiaca Regel;
– Canna lutea subsp. genuina Kraenzl.;
– Canna lutea subsp. maculata (Hook.) Petersen;
– Canna lutea subsp. pallida Kraenzl.;
– Canna lutea var. aurantiaca (Roscoe) Regel;
– Canna lutea var. genuina Kraenzl.;
– Canna lutea var. maculata (Hook.) Regel;
– Canna lutea var. pallida (Roscoe) Regel;
– Canna macrophylla Horan.;
– Canna maculata (Hook.) Link;
– Canna maxima Lodd.;
– Canna maxima Lodd. ex Roscoe;
– Canna montana Blume;
– Canna moritziana Bouché;
– Canna musifolia Année ex Chaté;
– Canna musifolia subsp. sanguinea Hend. & Andr.Hend.;
– Canna musifolia-edulis André;
– Canna musifolia-hybrida Année;
– Canna musifolia-hybrida Année ex André;
– Canna napalensis Wall. ex Bouché;
– Canna nepalensis Bouché;
– Canna nepalensis D.Dietr.;
– Canna occidentalis Ker Gawl.;
– Canna occidentalis KerGawl.;
– Canna occidentalis Roscoe, 1824;
– Canna orientalis Bouché;
– Canna orientalis Roscoe;
– Canna orientalis subsp. flava Roscoe;
– Canna orientalis subsp. flavescens (Link) Baker;
– Canna orientalis var. flava Roscoe;
– Canna orientalis var. flavescens (Link) Baker;
– Canna pallida Roscoe;
– Canna pallida subsp. maculata (Hook.) Roscoe;
– Canna pallida var. maculata (Hook.) Roscoe;
– Canna patens (Aiton) Roscoe;
– Canna patens subsp. limbata (Regel) Baker;
– Canna patens var. limbata (Regel) Baker;
– Canna pentaphylla D.Dietr.;
– Canna peruviana Année;
– Canna peruviana Année ex André;
– Canna peruviana-purpurea Année ex Chaté;
– Canna peruviana-robusta Année ex Chaté;
– Canna peruviana-spectabilis Année;
– Canna peruviana-spectabilis Année ex Chaté;
– Canna platyphylla Nees & Mart.;
– Canna plurituberosa T.Koyama & Nob.Tanaka;
– Canna poeppigii Bouché;
– Canna polyclada Wawra;
– Canna polymorpha Bouché;
– Canna polymorpha Lodd.;
– Canna polymorpha Lodd. ex Loudon;
– Canna porteana André;
– Canna portoricensis Bouché;
– Canna pruinosa Hoffmanns.;
– Canna pulchra Bouché;
– Canna pulchra Bouché ex Horan.;
– Canna pulchra Hassk.;
– Canna purpurea-spectabilis Année;
– Canna purpurea-spectabilis Année ex Chaté;
– Canna purpureaspectabilis Rob.;
– Canna recurvata Bouché;
– Canna rendatleri G.Nicholson;
– Canna robusta Année;
– Canna robusta Année ex André;
– Canna roscoeana Bouché;
– Canna rotundifolia André;
– Canna rubra Willd.;
– Canna rubricaulis Link;
– Canna sanctae-rosae Kraenzl.;
– Canna sanguinea Bouché;
– Canna sanguinea Warsz.;
– Canna sanguinea Warsz. ex Otto & A.Dietr.;
– Canna saturate-rubra Bouché;
– Canna saturate-rubra Bouché ex K.Koch;
– Canna schubertii Horan.;
– Canna seleriana Kraenzl.;
– Canna sellowii Bouché;
– Canna speciosa Hegetschw;
– Canna speciosa Roscoe;
– Canna speciosa Roscoe ex Sims;
– Canna spectabilis Bouché;
– Canna sulphurea Bouché;
– Canna surinamensis Bouché;
– Canna tenuiflora Bouché;
– Canna tenuiflora Bouché ex A.Dietr.;
– Canna texensis Regel;
– Canna textoria Noronha;
– Canna thyrsiflora Hegetschw.;
– Canna tinei Tod.;
– Canna tineoi Tod.;
– Canna vanhouttei Lierv.;
– Canna vanhouttei Lierv. ex André;
– Canna variabilis Willd.;
– Canna variegata Besser;
– Canna variegata Bouché;
– Canna variegatifolia Ciciar.;
– Canna ventricosa Bouché;
– Canna warszewiczii A.Dietr.;
– Canna warszewiczii subsp. flameus Ram.Goyena;
– Canna warszewiczii var. flameus Ram.Goyena;
– Canna xalapensis Bouché;
– Canna zebrina Année;
– Canna zebrina Année ex André;
– Canna zebrina subsp. nana André;
– Cannacorus indicus (L.) Medik.;
– Cannacorus ovatus Moench;
– Distemon brasiliensis (Roscoe ex Spreng.) Bouché;
– Distemon grandis Horan.;
– Xyphostylis lutea (Mill.) Raf..
The following varieties are recognized within this species:
– Canna lutea var. aurantiaca Kraenzl., 1912;
– Canna lutea var. aurantiaca Petersen, 1890;
– Canna lutea var. lutea;
– Canna lutea var. maculata Petersen, 1890;
– Canna lutea var. pallida Kraenzl., 1912;
– Canna lutea var. pallida Petersen, 1890.
Etymology –
The term Canna comes from the Latin canna referable to the Arundo (from arúndo, -dinis: reeds and rushes in general) and, by extension, to similar plants; the term is derived from the Greek κάννα kánna canna, perhaps from the Hebrew Qaneh, which could derive from the Akkadian qanûm.
The specific epithet indicates is in reference to India or Indie: because the first specimens of Canna introduced in Europe had been imported from the East Indies, although they originated in the Americas.
Geographic Distribution and Habitat –
Canna indica is native to South America and is present in: Colombia, Venezuela, Ecuador, Peru, Brazil, Uruguay and Argentina, as well as in the West Indies and Central America.
However this plant then naturalized in modern times in other countries, such as: Austria, Portugal, Spain, the Azores, the Canary Islands, Cape Verde, Madeira, a large part of tropical Africa, Ascension Island, Saint Helena, Madagascar, China, Japan, Taiwan, Bonin Islands, India, Nepal, Sri Lanka, Cambodia, Laos, Thailand, Vietnam, Burma, Java, Malaysia, Philippines, Christmas Island, Bismarck Archipelago, Norfolk Island, New South Wales, Queensland, Fiji, Tonga, Vanuatu, Kiribati, Cook Islands, Society Islands, Caroline Islands and Hawaii.
Its habitat is mainly that of vegetations growing in damp or wet places, or along streams, frequent in secondary growth, often invading cultivated lands, as in coffee plantations, at altitudes from sea level up to 1,900 metres.
Description –
Canna indica is a perennial herbaceous plant, with a fleshy and branched rhizome measuring 20 x 15 cm. The surface of the rhizome is incised by transverse furrows, which mark the scaly base. Small white roots rise from the lower part, and from the apex, where there are numerous buds, the leaves, the floral ensemble and the ramifications grow.
The aerial ramifications can reach 1-3 m in height and form a compact mass, being enveloped by the sheaths of the leaves.
The leaves are large, green or purplish green, with short petioles and elliptical laminae; they can measure from 30 to 60 cm in length and from 10 to 25 cm in width, with the broad base narrowing to a wedge. The apex is short, sharp and acute. The central rib is prominent, from which the lateral ribs depart.
The inflorescence has a terminal cluster and bears 6-20 groups of 1-2 flowers; the flowers have a 0,2-1 cm peduncle, red or yellow-orange, with the exception of some 4,5-7,5 cm varieties with triangular 1-1,7 cm sepals and erect petals of 4-6.5 cm. Tube 1.5–2 cm in size. 3-4 stamens, very oval and spatulate, 4,5-7,5 cm long and 0,3-0,5 cm broad in the free part.
The fruits are ellipsoid and globular shaped capsules, the surface is warty, 1,5 to 3 cm long, of chestnut color, with a large quantity of black and round and smooth seeds.
Cultivation –
Canna indica is a perennial plant that provides food (especially the root), medicines and a range of products. It is often cultivated on a domestic scale for these uses, especially in South America and South-East Asia; while it is grown on a small scale in Australia as a commercial source of root. The plant is widely grown in the tropics and subtropics as an ornamental plant, being valued mostly for its attractive flowers and leaves.
This plant can be grown from sea level up to 2,700 m above sea level, but thrives in temperate, tropical or subtropical montane climates, between 1,000 and 2,000 m above sea level (in humid tropical climates for higher areas ); the average temperature must be between 14 and 27 °C.
The plant prefers an average annual rainfall of between 1,000 and 4,500 mm but can tolerate 500–5,000 mm per year.
From a pedological point of view, it prefers light sandy-clayey soils, but it can also grow on heavy soils, provided they are not wet. It prefers a pH between 5.5 and 7.5, tolerating 5 – 8.
The plant has large leaves and does not like windy conditions as this can tear the leaves to shreds.
The plants grow rapidly and may produce a flowering shoot in the first year of growth from seed.
Rhizome cuttings develop into harvestable plants 6 – 8 months after sowing.
Plants grown from rhizome tips can be harvested 4 months after sowing, but harvesting after 8 months gives higher yields, because at that time the rhizomes are fully swollen. Rhizomes should not be allowed to age much longer than 10 months as they become hard and less suitable for consumption or starch production.
A rhizome is considered mature when the triangular fissure in the rhizome’s outer leaf has turned purple.
The rhizome yield varies from 23 tons per hectare at 4 months; at 45 – 50 tons at 8 months; to 85 tons after one year. Reported starch yields are 4-10 tonnes, exceptionally 17.5 tonnes per hectare.
Plants grown for ornamental purposes begin flowering a few months after planting in tropical regions, and flowers continue to appear as long as the plant lives. In cooler regions, where frost can be expected, the rhizomes should be lifted and overwintered at around 7°C.
This plant easily reproduces by division, but also by seed, which however must be previously scarified and left in water for a few days, given the hardness of its integument, to facilitate its germination.
In the propagation by seed it should be remembered that, since the different species of this genus often hybridize, one cannot rely on the fact that the seed reproduces the original plant correctly.
Canna indica is quite resistant to various plant diseases and diseases. However, it has been recorded that among the diseases that affect it, one of these is Canna rust (Puccinia thaliae), a fungus that causes orange spots on the leaves. In addition, plant viruses occur: Hippeastrum mosaic virus, tomato aspermia virus, canna yellow spot virus, and canna yellow streak virus that can cause mild to strong symptoms from streaked leaves, stunted growth, and distorted blooms. There is also Botrytis (fungus), a mold that affects flowers.
There are many different Canna varieties and some of them are resistant to a certain type of disease. To prevent mold, the soil must be well drained, without too much moisture or standing water. To decrease the risk of spreading disease, dead and infected leaves should be removed.
Pests include the Calpodes ethlius moth which has been observed on cane plants in the United States. This pest causes damage by laying eggs on the shoots of developing stems. To protect the eggs from predators and insecticides, caterpillars use sticky webs to keep the leaves from unfurling. The pupa then feeds on the leaves, which can lead to yield losses due to impaired photosynthesis.
Popillia japonica is also a leaf-damaging pest with mostly minor consequences for cannabis plants. This beetle feeds on the part of the leaves between the veins. In its home region of Japan, it doesn’t cause much damage.
Rhopalosiphum padi also affects stored rhizomes. Although this pest has not yet caused any serious damage, it can mostly affect plants grown in greenhouses and can be combated with parasitic wasps. It is a more common pest on cereals.
Please note that in some countries Canna indica is an invasive species. This plant has been included in the Global Invasive Species Database and has been declared invasive in the following locations:
South Africa, where it is classified as a category 1b invader in terms of the National Environmental Management List of Invasive and Alien Species: Biodiversity Act (10/2004) which prohibits their cultivation, propagation, translocation and trade and requires them to be removed and destroyed when found. This is because it competes with and replaces indigenous species, often in streams and marshy areas.
Australia, considered a weed in New South Wales and southeastern Queensland.
Pacific Islands, where it has been included in the list of plants threatening Pacific ecosystems, as a high risk species.
Tanzania, where although it was included in a list of 41 “problem” plants in the Serengeti-Mara ecosystem, it was assessed as naturalized in tourist areas, but non-invasive (using roadside surveys).
Ghana, where it has been noted to compete with and invade the natural vegetation of scrub and woodland in the Boabeng-Fiema Monkey Sanctuary and Kakum National Park.
Customs and Traditions –
Canna indica is a widespread plant in the neotropical ecozone and in particular in the Caribbean and central areas of America.
Due to its widespread diffusion, this plant is known by various names, including: “indian-shot”, “canna”, “wild canna lily” (English); “chupa flor”, “achira”, “bandera de uriba”, “caña edible”, “caña de la india”, “caña coro”, “café cimarrón”, “capacho”, “chisgua”, “chumbima”, “ cucuyús”, “lengua de dragón”, “maraca”, “papantia”, “platanillo”, “yuquilla”, “sagú” (Spanish); “conflore”, “balisier à chapelets”; “balisier des indes”, “balisier rouge, “canna”, “canna florifére”, “canne d´inde”, “toloman”, “tous-les-mois”, “faux sucrier”, “balisier comestible” (French) ; “albará”, “araruta bastarda”, “araruta de porco”, “bananeirinha-da-índia”, “bananeirinha-de-flor”, “beri”, “birù manso”, “caeté-dos-jardns”, “cana -da-índia”, “erva-de-conteira” (Portuguese); “westindisches blumenrohr” (German). In the lands of origin it is known above all by the names of achira, achera, sagú, capacho, biri, cucuyús, juquián or papantla.
The rattan is also known in Colombia as sagú or chisgua, as capacho or maraca in Venezuela, as achera or atzera (or atcera) in Peru and Ecuador, and as biri in Brazil. Other denominations are chui’o arawak imocoma.
The plant was introduced in Europe in the second half of the 1500s and quickly spread also in Africa and Asia. In addition to being cultivated as an ornamental plant, even if for this purpose the botanical species has now been replaced by its very numerous hybrids and varieties with large showy inflorescences of various colors, its rhizomes have had great importance in human and animal nutrition in ancient times and still today in some tropical and subtropical areas the rhizomes, rich in highly digestible starch, are eaten cooked in the oven or grilled, while the starch is used in confectionery. The rhizomes are also variously used in traditional medicine, while necklaces and rosaries are made with the very hard seeds.
Archaeologists have discovered that it was grown in Peru 5,000 years ago in the Sacred City of Caral, America’s first civilization. The ancient Caralinos domesticated it and used its roots as part of their daily diet; its medicinal use has also been developed for its diuretic, antiseptic, analgesic and healing properties. In Colombia the chibcha used it in their diet. Currently in Colombia, through rural agro-industry processes, achira starch is extracted, which in turn is used to make achira biscuits and other artisanal products such as biscuits, sago bread, snacks and coladas. In the departments of Tolima, Huila and Cundinamarca in Colombia, a large number of small rallanderías have sprung up dedicated to the extraction of starch and various artisanal and industrial companies dedicated to the production of the achira cake, which is gaining more and more acceptance in the urban markets.
In Colombia the chibchas were used for food. Currently, through rural agro-industry processes, Indian cane starch is extracted, which in turn is used for the preparation of Indian cane biscuits and other handicraft products such as biscuits, jaggery bread, breakfasts and purées . In the departments of Tolima, Huila y Cundinamarca, in Colombia, a large number of small companies have arisen dedicated to the extraction of starch and various artisanal and industrial companies dedicated to the production of the cane biscuit, which is spreading in the markets urban.
Today we know that canna root indica is rich in carbohydrates, ascorbic acid, vitamin A and minerals such as calcium, iron, potassium and magnesium.
The rhizomes can be eaten raw, but are usually cooked in the oven. When cooked, the rhizomes become translucent, mucilaginous and sweet. Starch is made by grinding or mashing the roots and soaking them in water, separating the starch granules from the root fibers. The starch is easily digested, and the flour is used to make bread, biscuits, cookies, cakes, noodles and noodles. In the Paria Peninsula, Venezuela, the flour is used to prepare an atol for children after weaning, and for the elderly and convalescents due to its high nutritional value. Corms of achira are also eaten roasted or cooked.
The roots are used to prepare diuretic decoctions and the leaves as a healing agent; the juice of these as an antiseptic. The freshly cut leaves were used, and are probably still used on burns to cool and dissipate heat from the burned skin. The seeds are used to make necklaces and rattles or maracas. Stems and leaves serve as fodder for livestock. The leaves are also used as a sort of natural packaging to wrap typical sweet-tasting foods similar to tamales, known as quimbolitos. In the rural area of the auctioneer called Laguna de García, in the state of Táchira, they are used to cover the emblematic hallacas instead of banana leaves.
In the canton of Girón, in the southern part of Ecuador, it is grown as a household product; however, it is a product with great potential because it derives from starch flour to make Dulce de Almidón, a kind of bread, with excellent acceptance in countries such as the United States, Spain and Italy, countries with Ecuadorian immigrants. That is why the indigenous people of this area which belongs to the province of Azuay are known as “achiras”.
The name “achira” comes from the Quechua term Achuy, whose primary meaning is “to sneeze”. It refers to the idea of “carrying something between the teeth or with the mouth” and from here to the concept of what the human soul emits or expresses spontaneously. For this reason the term achira indicates “the word”, the “story”, the “story” and is connected to the transmission of oral knowledge. It can be found in terms such as Arachán an extinct family native to eastern Uruguay and southern Rio Grande in Brazil, and also in the name of the border town of Chuy, located between these two countries.
In Europe it is mainly used as an ornamental plant in gardens, whilst in Latin America it is mainly cultivated for its corms or rhizomes, which are important for human consumption and agro-industry.
The rattan is an ornamental plant that has long been used in gardens due to its spectacular appearance, its resistance and the scarcity of necessary maintenance. Despite this, it has been used less and less in Italy in recent decades, giving preference to species introduced more recently in gardens.
Among the historic parks where rattan is still used in large quantities, we mention Villa Margherita in Trapani (Sicily), the Park of the Italian Embassy in Addis Ababa; in the famous Villa Taranto there are 10,000 specimens of this species.
It should be remembered that while the spontaneous Canna indica has green leaves and red flowers, there are numerous ornamental varieties with different colors.
Among other uses, it should be remembered that the plant produces a fiber that can be a substitute for jute. Furthermore, a fiber obtained from the leaves is used to make paper.
The leaves are harvested in late summer after the plant has flowered, scraped to remove the outer skin and then soaked in water for 2 hours before cooking. The fibers are cooked for 24 hours with lye and then beaten in a blender. They produce a light brown paper.
The large leaves are sometimes used as dishes.
A purple but not very permanent dye is obtained from the seed.
The smoke from the burnt leaves is said to be insecticide.
The hard seeds are used for various purposes as well as grains for making rosaries and necklaces.
Method of Preparation –
Canna indica is a plant used since ancient times for food, medicine and other purposes, in the countries of origin.
Its starch is easy to digest and the flour is used to make bread, biscuits, biscuits and cakes. The tops of the rattan cane can be eaten stewed or boiled. The decoction of the roots is used as a diuretic and the leaves as a healing agent; the juice of these is used as an antiseptic. The freshly cut leaves are used on burns to refresh the skin. The thallus and leaves serve as fodder for livestock. The leaves are also used to wrap typical food.
In the countries where it has been exported, it is instead used above all as an ornamental plant.
Among the edible uses we remember that the root is sometimes eaten raw, but usually consumed after being cooked in various ways. The very young tubers are eaten cooked, they are sweet but fibrous.
In Peru they are cooked for up to 12 hours, after which they become a white, translucent, fibrous and somewhat mucilaginous mass with a sweet taste.
The roots contain about 25% starch.
The roots are the source of “cane starch”, which is used as arrowroot.
It is obtained by scraping the root until it is reduced to a pulp, washing it and filtering it to remove the fibers and then drying it.
Alternatively, the roots can be peeled, dried and then ground into flour.
Flour is over 90% starch and about 10% sugar (glucose and sucrose). The starch produced is a shiny yellowish powder with very large grains (125 – 145 µm 60 µm) of irregular shape. It is highly soluble and easily digested. After cooking, the starch is shiny and transparent.
The young shoots are cooked and eaten as a green vegetable.
The leaves are used to wrap other foods.
The immature seeds are cooked into the tortillas.
In medicinal use the plant is used in the treatment of women’s ailments.
The root is diaphoretic and diuretic.
It is used in the treatment of fevers.
A decoction of the root, combined with fermented rice, is used in the treatment of gonorrhea and amenorrhea.
An infusion of the rhizome is said to be febrifuge and stimulant, while the decoction is diaphoretic and diuretic.
An emollient poultice is also made from the rhizome.
The leaves are diuretic and emollient.
The pulverized leaves and seeds are mixed and used to treat dermatoses.
The seeds are emollients. They are mixed with water in a poultice that is placed on the forehead to remedy headaches. They are ground into a powder and used as an anti-infective agent or as a treatment for itching, persistent sores and “yaws”.
Guido Bissanti
Sources
– Acta Plantarum – Flora of the Italian Regions.
– Wikipedia, the free encyclopedia.
– GBIF, the Global Biodiversity Information Facility.
– Useful Tropical Plants Database.
– Conti F., Abbate G., Alessandrini A., Blasi C. (ed.), 2005. An annotated checklist of the Italian vascular flora, Palombi Editore.
– Pignatti S., 1982. Flora of Italy, Edagricole, Bologna.
– Treben M., 2000. Health from the Lord’s Pharmacy, Advice and experiences with medicinal herbs, Ennsthaler Editore.
Photo source:
– https://inaturalist-open-data.s3.amazonaws.com/photos/211231786/original.jpeg
Attention: The pharmaceutical applications and alimurgical uses are indicated for informational purposes only, they do not in any way represent a medical prescription; we therefore decline all responsibility for their use for curative, aesthetic or food purposes.