Coumaric acid
Coumaric acid
Coumaric acid is an acid with a brute or molecular formula: C9H8O3.
Coumaric acid together with ferulic acid, caffeic acid, chlorogenic acid, rosmarinic acid and synapic acid are part of the hydroxycinnamic acids.
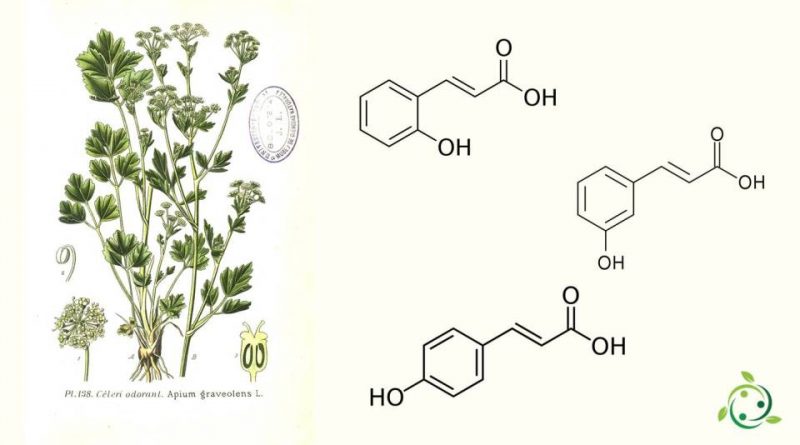
This compound is a hydroxy derivative of cinnamic acid and depending on the position of the -OH group it can be in the form of the three isomers, namely:
– ortho-coumaric acid;
– meta-coumaric acid;
– para-coumaric acid.
P-coumaric acid is the most present acid in nature and we find it, for example in celery (Apium graveolens L.) and hollyhock (Alcea rosea L., 1753).
Due to the presence of the double bond, it can in turn present itself in the E and Z form.
Coumaric acid is typically synthesized from the hydroxylation of cinnamic acid. With other hydroxycinnamic acids, its esters with quinic acid, oxidizing, take on a green color and therefore would belong to the family of chlorogenic acids.
P-coumaric acid reduces oxidative stress and inflammatory reactions. It is an energetic antioxidant and fights the formation of free radicals. It is also used for the control of hyperpigmentation. In fact, it is a competitive inhibitor of tyrosinase, an enzyme capable of converting the amino acid tyrosine into melanin.
Warning: The information shown is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.