Anthranilic Acid
Anthranilic Acid
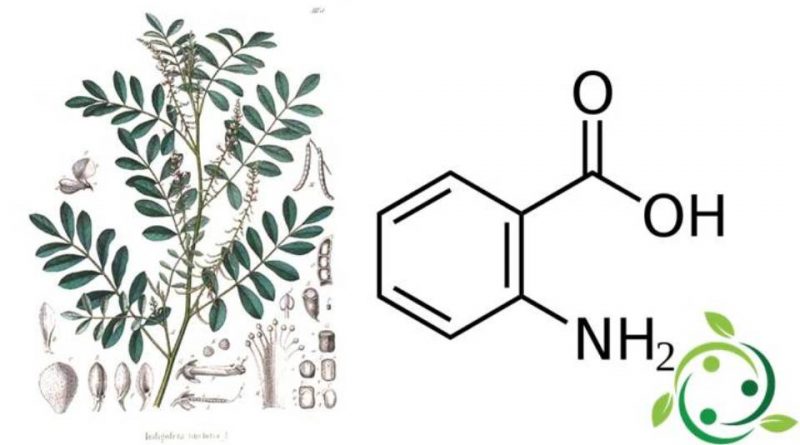
Anthranilic acid, whose term in the official IUPAC nomenclature is: 2-aminobenzoic acid, also known by the alternative names of ortho-aminobenzoic acid or o-aminobenzoic acid, is an aromatic acid with a brute or molecular formula: C7H7NO2.
Anthranilic acid, although not usually referred to as such, is an amino acid. Solid anthranilic acid consists of both amino carboxylic acid and the carboxylate forms of zwitterionic ammonium.
The anthranilic acid molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine.
Due to the various functional groups both acidic and basic, the compound is amphoteric.
Anthranilic acid, at room temperature, appears as a yellow solid, odorless and not very soluble in water. In addition, anthranilic acid has a sweetish taste.
The anion obtained from the deprotonation of anthranilic acid is called anthranilate.
On an industrial level, anthranilic acid is produced from phthalic anhydride, starting from the amination:
C6H4 (CO) 2O + NH3 + NaOH → C6H4 (C (O) NH2) CO2Na + H2O
The resulting sodium salt of phthalamic acid is decarbonylated by a Hofmann rearrangement of the amide group, induced by hypochlorite:
C6H4 (C (O) NH2) CO2Na + HOCl → C6H4NH2CO2H + NaCl + CO2
A related method involves treating phthalimide with sodium hypobromite in aqueous sodium hydroxide, followed by neutralization.
In the days when indigo dye was obtained from plants, it was degraded to give anthranilic acid.
In fact, anthranilic acid was obtained for the first time from the degradation induced by indigo bases.
At the biochemical level, anthranilic acid is biosynthesized starting from chorismic acid. In organisms capable of synthesis of tryptophan, anthranilate is a precursor of the amino acid tryptophan, through the attachment of phosphoribosyl pyrophosphate to the amino group.
On an industrial level, anthranilic acid is an intermediate in the production of azo dyes and saccharin.
It and its esters are used in the preparation of fragrances that mimic jasmine and orange, pharmaceuticals and products that absorb UV rays, as well as metal corrosion inhibitors and mold inhibitors in soy sauce.
In addition, some insect repellents, based on anthranilate, have been proposed as substitutes for DEET (Dietiltoluamide).
Anthranilic Acid is an amino acid and acts as a coenzyme in the production of breast milk. It therefore represents a metabolite of Tryptophan, another important amino acid crucial for the well-being of the neuropsychic system and its maintenance in health.
Since it performs the function of coenzyme in the production of breast milk, it is therefore also called vitamin L according to an obsolete nomenclature of vitamins.
In fact it was once thought that anthranilic acid was a vitamin and in that context it was called vitamin L1, but it is now known that it is not essential in human nutrition.
Warning: The information shown is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.