Glucuronic acid
Glucuronic acid
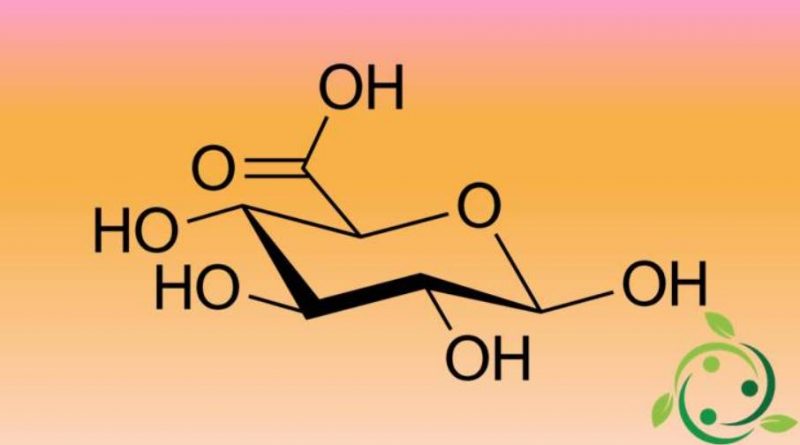
Glucuronic acid (more precisely D-glucuronic acid), whose brute or molecular formula is: C6H10O7, is an organic acid of the family of alduronic acids. The levorotatory form of glucuronic acid is iduronic acid (L-iduronic acid).
Glucuronic acid is produced by the oxidation of the primary alcohol group (OH bound to C-6) of D-glucose to carboxyl group.
Inside its molecule the glucuronic acid has the three different functional groups: OH, -CHO, -COOH, so it is an aldoxyacid.
The biological importance of glucoronic acid is to be identified in the detoxification mechanisms operated inside the animal cells.
Glucuronidation is the process of binding a generally toxic or xenobiotic molecule to one of the molecule’s alcohol groups. Many cells, especially hepatocytes, exploit this mechanism to make foreign substances, such as antibiotics or small toxins, more soluble, and expel them through kidney function.
In terms of metabolism, steroid hormones (insoluble in water) are found in the blood and urine mainly as glucuronides (glucuronic acid glucosides). Phenol, which is toxic to humans, is also eliminated as glucuronide because of its greater solubility in water.
Warning: The information given is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.