Borneol
Borneol
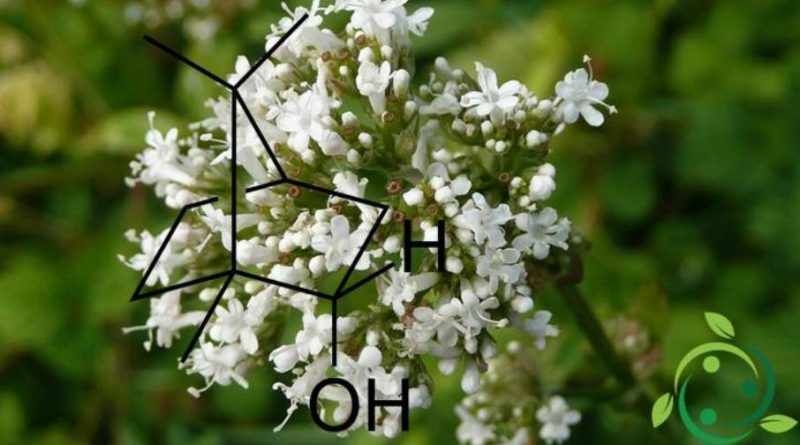
Borneol, whose term in the IUPAC nomenclature is: endo-1,7,7-Trimethyl-bicyclo [2.2.1] heptan-2-ol and whose brute or molecular formula is: C10H18O, is a bicyclic organic compound of the family of terpenes. In the borneol the hydroxyl group is placed in the endo position.
There are two optical isomers of this molecule of which the predominant is the form d. Isoborneol is the corresponding isomer. The enantiomer present in nature is right-handed.
From the oxidation of borneol the corresponding ketone is obtained, the camphor, known with one of its historical names is namely: “camphor of Borneo”.
In nature, borneol is present in many plant species including: Teucrium chamaedrys, Satureja montana, Juniperus communis, Cannabis sativa, Coriandrum sativum, Tanacetum vulgare, Rosmarinus officinalis, Centranthus ruber, Achillea millefolium, Artemisia arborescens, Valeriana officinalis, in plants of this genus Cymbopogon (Citronella) and some of their respective essential oils.
Moreover, among its interesting features we recall that borneol is an insect repellent.
In the laboratory, borneol can be synthesized by reduction of camphor according to the Meerwein-Ponndorf-Verley reaction. Isoborneol is obtained from the reduction of camphor with sodium borohydride.
Borneol is used in traditional Chinese medicine. It is anciently mentioned in the Bencao Gangmu, the Chinese herbalist treatise written by Li Shizhen during the Ming dynasty.
Inappropriate intake of borneol can cause nausea, confusion, dizziness and convulsions.
Warning: The information given is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.