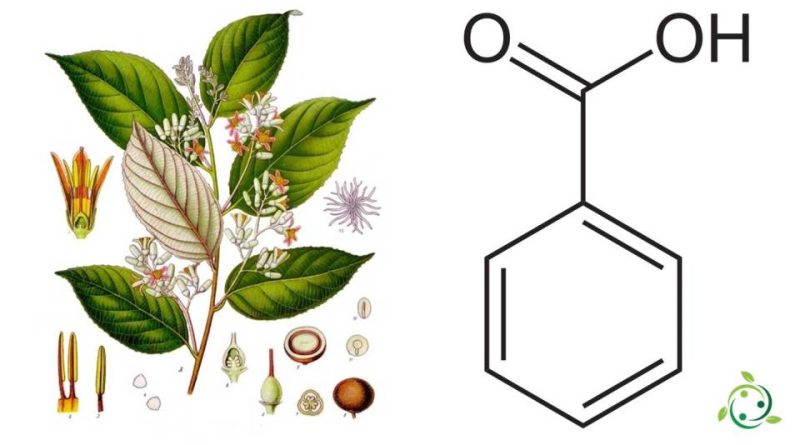
Benzoic acid
Benzoic acid
Benzoic acid, known by the abbreviation E210, is an aromatic carboxylic acid having the brute or molecular formula: C7H6O2.
Benzoic acid is a white crystalline compound, in powdery or granular form, slightly soluble in cold water, more in hot water; it is soluble in organic solvents. It melts at 122°C and boils at 250°C; it is stable up to 370°C, but above this temperature it begins to decompose into benzene and CO2, also supplying phenol (0.2-0.8%).
Being a weak acid, its aqueous solution is weakly acidic.
History –
Benzoic acid was discovered in the sixteenth century. The dry distillation of benzoin gum was first described by Nostradamus (1556), and then by Alessio Piemontese (1560) and Blaise de Vigenère (1596).
We have to get to 1830 when Pierre Robiquet and Antoine Boutron-Charlard, two French chemists, thanks to experiments on amygdalin, obtained from bitter almonds, produced benzaldehyde, but they failed to give a correct interpretation of the structure of amygdalin which contains the radical benzoyl.
In 1832, Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid, also studying its correlation with hippuric acid.
In 1875 Salkowski discovered the antifungal capabilities of benzoic acid, which was used for a long time in the preservation of blueberry fruits.
The salts and esters of benzoic acid are known as benzoates, and are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances.
In nature, benzoic acid is found in many plants and serves as an intermediate in the biosynthesis of secondary metabolites.
The name benzoic acid comes from the benzoin resin which has long been its only known source, which contains up to 20% benzoic acid and 40% benzoic acid esters; benzoin resin is a balsamic resin obtained from the bark of the Styrax benzoin Dryand plant. and also present in some types of fruit, for example in blueberries and plums, in the balsam of Peru and in Tolù, in other balms, in yogurt, but in practice it is produced by chemical synthesis starting from phthalic anhydride or preferably from from toluene which is an aromatic hydrocarbon.
Properties and uses –
Benzoic acid inhibits the growth of yeasts and molds creating an antimicrobial effect, for this reason it is used as a food preservative and in many cosmetic preparations, such as lotions, creams, hair products and deodorants, always in association with other antimicrobial substances.
It is highly tolerated on the skin, with a low environmental impact.
Benzoic acid and its salts are used as food preservatives to combat the action of yeasts and bacteria in foods with an acid pH; they are usually used in flavored soft drinks, alcoholic beverages, fish preserves, jams, most fruits, especially blueberries, mushrooms, cinnamon, cloves and some dairy products.
At the concentrations used, there appear to be no adverse health effects; however, it may happen that in predisposed subjects, benzoic acid and benzoates can trigger allergic reactions.
Their use in various products is limited, because if present in high concentrations they produce a bitter taste.
Warning: The information provided is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.