Glucosamine
Glucosamine
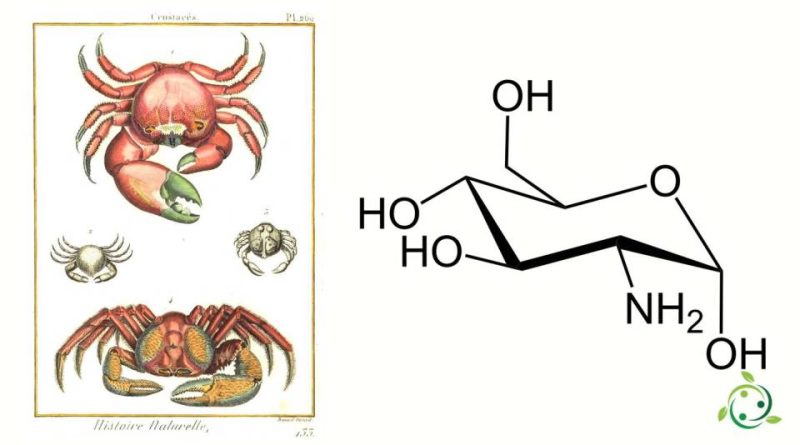
Glucosamine whose term in the official IUPAC nomenclature is: (3R, 4R, 5S, 6R) -3-amino-6- (hydroxymethyl) oxan-2,4,5-triol is an aminomonosaccharide and one of the main precursors of the synthesis of glycosylated proteins and lipids.
Glucosamine, also known as: 2-amino-2-deoxy-D-glucose or chitosamine has a molecular or brute formula: C6H13NO5.
This organic molecule is one of the major components of the shell of crustaceans and other arthropods, fungi and many higher organisms. It is also one of the components of the lipopolysaccharide of Gram-negative bacteria.
In organic chemistry, however, the term glucosamine indicates any member of the aminodeoxyglucose class of regioisomers even if, almost invariably, it implies 2-D – (+) – glucosamine (2-amino-2-deoxy-D-glucose, or chitosamine), which is by far the most important member of the family and which shares the steric series D with glucose (and the vast majority of sugars).
Glucosamine is not a monosaccharide in the strict sense of the term, as its molecular formula does not correspond to the general formula CnH2nOn. 2-D-glucosamine is the unit that is repeated, by means of (1β → 4) glycosidic bonds, in the chitosan polysaccharide and also, in the form of N-acetylamide, in chitin. These polymers make up the exoskeletons of crustaceans and the cell walls of fungi and other higher organisms. It is therefore one of the most widespread abundant carbohydrate units. It is commercially produced by the deep hydrolysis of crustacean exoskeletons.
History –
Historically 2-D-glucosamine was first prepared and identified in 1876 by G. Ledderhose although the stereochemical configuration was not fully defined until 1939 with the work of W. Haworth.
Glucosamine has been studied since 1969 as a therapeutic agent in osteoarthritis. Initially, pain relief was found in some uncontrolled studies following the administration of glucosamine sulfate by injection (iv, ia, i am). However, clinical research did not go on until the early 1980s, when the Italian pharmaceutical company Rottapharm Milano tablets glucosamine sulfate. Virtually all glucosamine research in the 1980s and 1990s was also done with tablets of this brand. Rottapharm has patented an extraction method to extract glucosamine from shellfish exoskeleton.
Physical-chemical –
Like all monosaccharides and their hemiacetal derivatives, glucosamine, in its crystalline state, is present as a mixture of the two optically active epimers (called anomers), both of which are dextrorotatory. The α and β forms have optical rotation [α] D20, respectively of + 100 ° and + 28 ° and in solution they mutarotate, with the stabilization of the optical rotation of the aqueous solutions at + 47.5 °.
From a chemical-physical point of view, 2-D-glucosamine is a substance with basicity (pKa = 7.8) lower than that of ammonia and common aliphatic amines, and is marketed in the form of stable glucosammonium salts.
From glucosamine, by chemical or enzymatic acetylation, N-acetylglucosamine is obtained, the basic unit of the natural polymer of chitin condensation.
Biochemistry –
Glucosamine is a molecule naturally present in the body; more precisely, it is part of the polysaccharide chains of the glycosaminoglycans of the cartilage matrix (including hyaluronic acid) and of the synovial fluid.
Glucosamine and the molecules in which it belongs as a component are involved in the structural activity and in maintaining the functionality of tissues subjected to continuous stress, such as those of the joints.
It is precisely the role played by glucosamine in the joints and cartilage that has awakened interest in this molecule.
In addition to being part of the composition of the glycosaminoglycans of the cartilage matrix, some studies have shown that glucosamine is able to favor their synthesis by chondrocytes, as well as stimulating the synthesis of proteoglycans, always by chondrocytes, and of favor the synthesis of hyaluronic acid by the synoviocytes. In addition to this, it seems that this amino-monosaccharide is able to exert even a modest anti-inflammatory activity. All these activities, therefore, should safeguard the functionality of the joints while preserving their integrity.
Property –
Several studies have demonstrated the ability of glucosamine to stimulate the synthesis of glycosaminoglycans and proteoglycans and to exert beneficial actions in the presence of diseases affecting the joints and cartilage. In particular, some clinical trials have shown that the administration of glucosamine can reduce pain and inflammation in patients suffering from osteoarthritis, improve joint mobility of the affected areas and reduce the amount of anti-inflammatory drugs administered to control this type of pathology.
Despite this, if on the one hand several studies confirm the properties of this molecule, on the other hand there are many who have doubts about the effectiveness of glucosamine supplements and believe that the use of similar products should be considered more than anything else. as an adjuvant remedy the traditional therapeutic strategies implemented in the presence of the aforementioned joint pathologies.
Furthermore, it should not be forgotten that the studies confirming the properties of glucosamine were conducted using certain concentrations of the molecule, with a well-defined frequency of administration and duration of treatment.
Contraindications –
Although glucosamine is a molecule naturally present in the body, taking this molecule, through supplements that contain it, is not without risks.
First of all, the glucosamine contained in these products is obtained from the exoskeleton of small crustaceans, this characteristic makes these supplements contraindicated in individuals with allergies to this type of animals.
Furthermore, the intake of glucosamine could give rise to undesirable effects, especially of a gastrointestinal nature, as well as it could cause interactions with drugs, such as hypoglycemic agents. In this regard, it should be remembered that supplementation with this amino-monosaccharide is contraindicated in patients with type 2 diabetes or with metabolic syndrome.
The intake during pregnancy and lactation, however, is not recommended due to the lack of reliable data regarding the safety of use.
In any case, it reiterates the importance of contacting your doctor before taking glucosamine supplements.
Warning: The information shown is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.