α-terpineol
α-terpineol
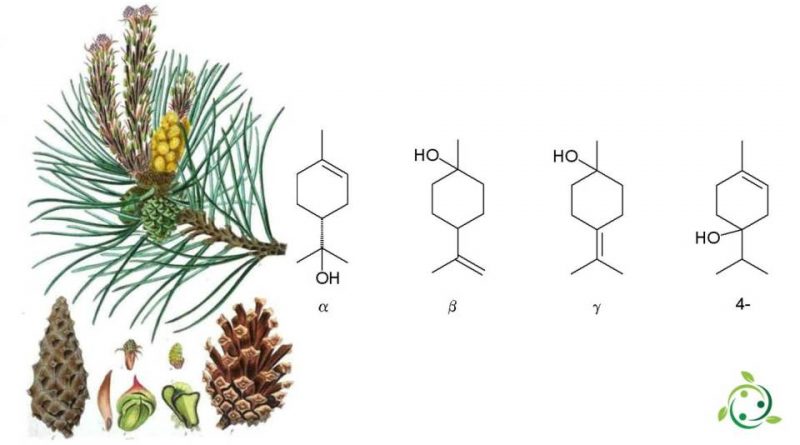
Α-terpineol, whose term in the official IPAC nomenclature is: 2- (4-Methyl-1-cyclohex-3-enyl) propan-2-ol is a natural monoterpenic alcohol with a brute or molecular formula: C10H18O.
Α-terpineol is a compound isolated from a variety of sources such as pine essential oil and petitgrain oil. Its presence is generally accompanied by small percentages of the two isomers β-terpineol and γ-terpineol. The natural blend of the three isomers take the generic name of terpineol.
Terpenoids are terpenes modified by the addition of a functional group, which in this case is an alcohol. There are four isomers: α-, β-, γ-terpineol and terpinen-4-ol. Terpineol is usually a mixture of these isomers with α-terpineol as the main constituent.
Terpineol has a pleasant lilac-like odor and is a common ingredient in perfumes, cosmetics, and flavorings.
Although this substance occurs naturally, it is commonly produced from the more readily available alpha-pinene. An alternative path of synthesis is that which starts from limonene.
In synthesis starting from limonene, the latter reacts with trifluoroacetic acid, according to the Markovnikov rule, arriving at an intermediate trifluoroacetate, which is easily hydrolyzed with sodium hydroxide to α-terpineol with a selectivity of 7%. The side products are β-terpineol in a mixture of the cis isomer, the trans isomer and the 4-terpineol.
The biosynthesis of α-terpineol instead proceeds from geranyl pyrophosphate, which releases pyrophosphate to give the terpinyl cation. This carbocation is the precursor of many terpenes and terpenoids. Its hydrolysis gives terpineol.
Warning: The information shown is not medical advice and may not be accurate. The contents are for illustrative purposes only and do not replace medical advice.